Switching from a brand-name drug to a generic version seems simple: same active ingredient, lower cost, right? But for some medications, even a tiny change in how the drug is made can throw a patient off balance. When doctors change doses after switching to generics, it’s not because they’re being cautious for no reason - it’s because the science says some drugs demand it.
Why Some Generics Need Dose Changes
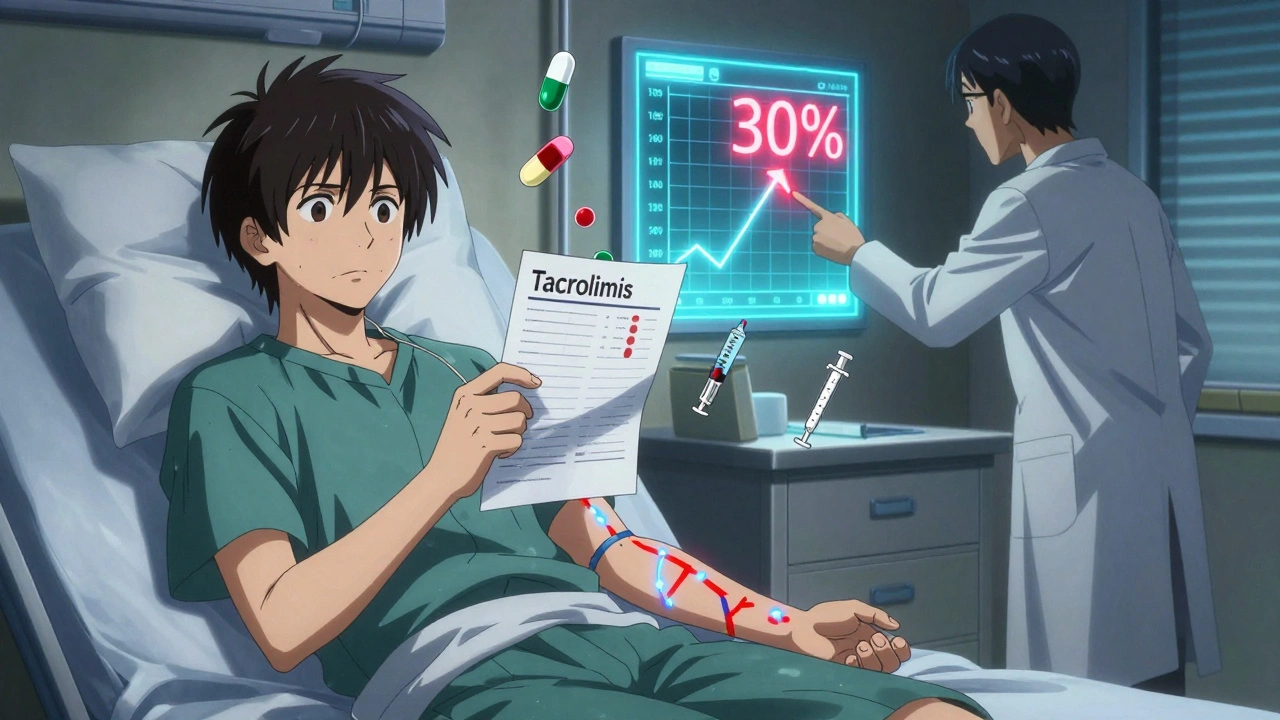
Not all generic drugs are created equal when it comes to how your body handles them. For most medications, a 10% difference in absorption won’t matter. But for drugs with a narrow therapeutic index (NTI), even a 5% shift can mean the difference between working properly and causing harm. NTI drugs have a very small window between the dose that works and the dose that’s dangerous. Think of it like walking a tightrope - a tiny stumble can lead to a fall. Examples include warfarin (a blood thinner), levothyroxine (for thyroid issues), phenytoin (for seizures), and tacrolimus (for organ transplant patients). These aren’t just any pills. They’re life-critical. The FDA says generics must be bioequivalent to the brand - meaning they deliver 80% to 125% of the same amount of drug into the bloodstream. That sounds broad, but for NTI drugs, that range is wide enough to cause problems. A patient stabilized on 5 mg of brand-name tacrolimus might need 5.75 mg of a generic version to get the same blood level. That’s not a mistake. That’s chemistry.Real Cases: When Switching Goes Wrong
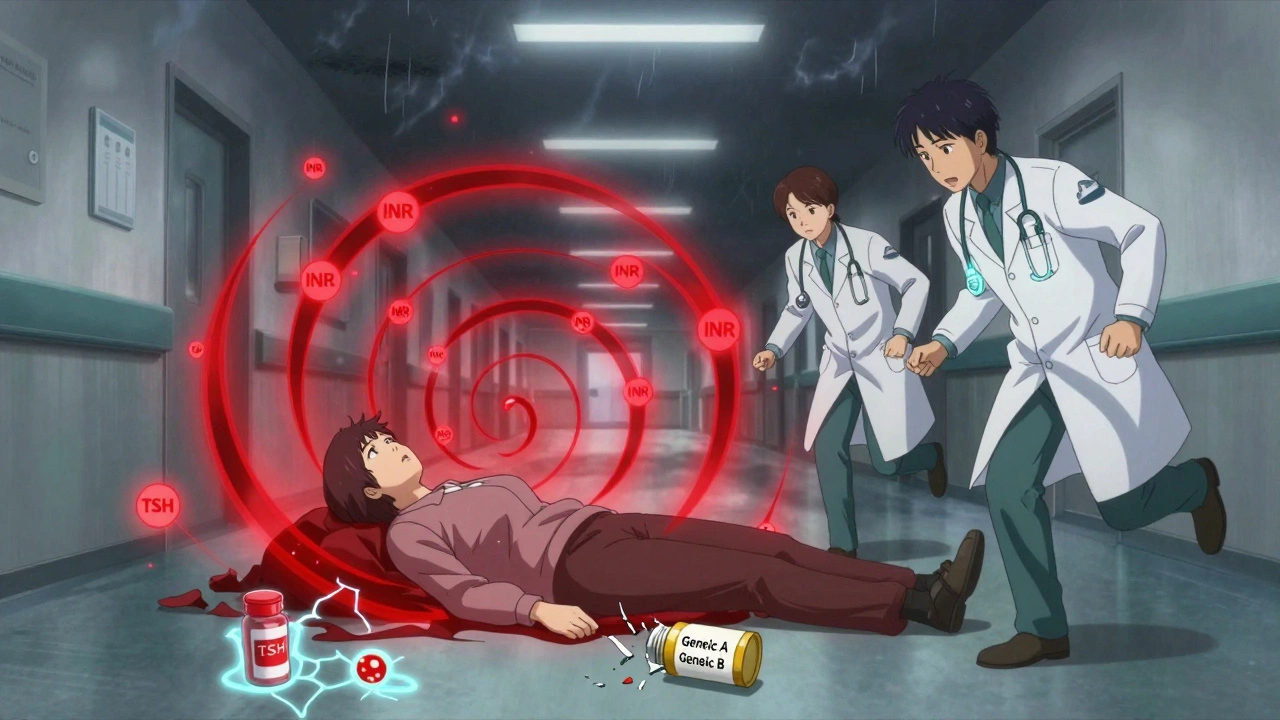
In 2022, a hospital in Ohio reported that 18 out of 45 transplant patients needed dose adjustments within two weeks of switching from brand to generic tacrolimus. One patient’s blood level dropped 30%. Within days, her body started rejecting the new kidney. She didn’t have symptoms until it was too late. Another case: a 62-year-old woman on warfarin for atrial fibrillation switched from one generic to another. Her INR - a measure of how long her blood takes to clot - jumped from 2.5 to 4.8 in 10 days. She ended up in the ER with a brain bleed. Her doctor hadn’t changed her dose. Neither had her pharmacist. But the generic formulation changed. Even levothyroxine, the most common NTI drug, causes issues. A 2023 study found that 31% of patients switching between different generic brands of levothyroxine had abnormal TSH levels within 6 weeks. Some felt exhausted, gained weight, or developed depression. Others had heart palpitations. All of them had the same prescription. Just a different pill.What Doctors Do About It
Most doctors don’t automatically change doses when a generic is switched. But for NTI drugs, they know they need to watch closely. Here’s what typically happens:- For warfarin: INR is checked within 7-14 days after the switch. If it’s more than 10% off the target, the dose is adjusted.
- For levothyroxine: TSH is retested at 6-8 weeks. A change of more than 0.5 mIU/L often means a dose tweak.
- For phenytoin: Blood levels are drawn within 10-14 days. A 20% drop or rise triggers a change.
- For tacrolimus: Levels are checked at days 3, 7, and 14. Any deviation over 15% leads to a dose adjustment.
Why This Isn’t Always Done
You’d think every doctor would check blood levels after a switch. But they don’t always. Why? First, many patients feel fine. They don’t have symptoms. So the doctor assumes it’s working. But with NTI drugs, symptoms often appear only after damage is done. Second, insurance companies push for the cheapest generic. Sometimes, a patient gets switched twice in a year - brand to Generic A, then Generic A to Generic B. Each switch carries risk. A 2022 survey found that 44% of pharmacists had to switch NTI drugs because of payer rules, not clinical need. Third, not all doctors know which drugs are NTI. Many think “generic = same.” They don’t realize that levothyroxine, digoxin, and carbamazepine are in a different category than, say, ibuprofen or metformin.What You Can Do
If you’re on a high-risk medication, here’s what to do:- Ask your doctor: “Is this a narrow therapeutic index drug?”
- Ask your pharmacist: “Which generic brand am I getting? Is it the same one as last time?”
- If you switch generics, ask for a blood test 4-6 weeks later - even if you feel fine.
- Keep a log: Note how you feel - energy, mood, heart rate, sleep - after any switch.
- If you notice changes, don’t wait. Call your doctor. Don’t assume it’s “just stress.”
The Bigger Picture
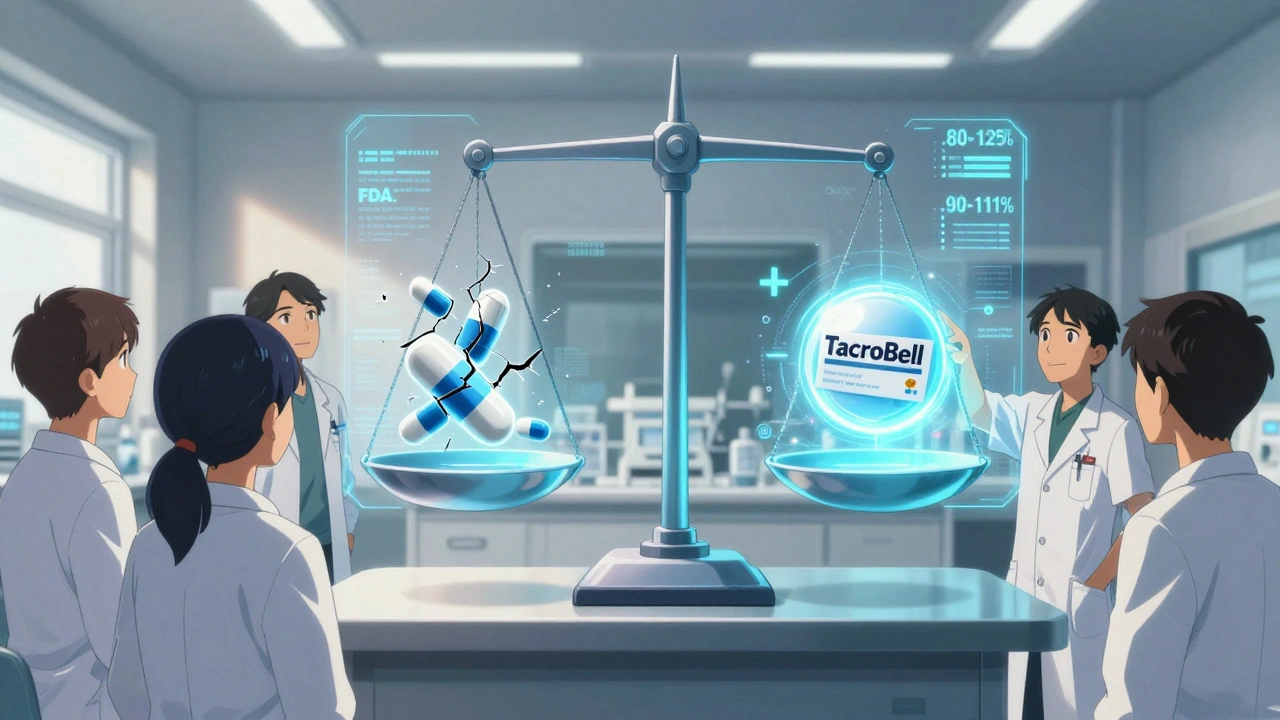
The FDA is aware of the issue. In 2023, they proposed new rules: tighten the bioequivalence range for NTI drugs from 80-125% to 90-111%. That’s a big deal. It means generics for warfarin or levothyroxine will have to be much more consistent. Some manufacturers are already responding. Teva’s “TacroBell” tacrolimus, for example, has 32% less variability than standard generics. It’s not cheaper, but it’s more predictable. The goal isn’t to stop generics. It’s to make sure the ones that matter most - the ones that can kill you if they’re off - are as safe as possible.Bottom Line
Switching to generics saves money. That’s good. But for a small group of critical drugs, that savings can come with hidden costs: hospital visits, complications, even death. Doctors adjust doses after switching generics not because they distrust generics - they do it because they’ve seen what happens when they don’t. If you’re on one of these drugs, don’t assume everything’s fine just because the pill looks different. Stay informed. Stay vigilant. Your life might depend on it.Do all generic drugs need dose adjustments after switching?
No. Only drugs with a narrow therapeutic index (NTI) require careful monitoring and possible dose changes. These include warfarin, levothyroxine, phenytoin, tacrolimus, digoxin, and a few others. For most common medications - like statins, antibiotics, or blood pressure pills - switching generics is safe without any dose changes.
How do I know if my medication is a narrow therapeutic index (NTI) drug?
Ask your doctor or pharmacist directly. You can also check the FDA’s Orange Book online, which lists drugs with special bioequivalence requirements. Common NTI drugs include levothyroxine (for thyroid), warfarin (blood thinner), phenytoin and carbamazepine (seizure meds), cyclosporine and tacrolimus (transplant drugs), and digoxin (heart medication). If your drug is on that list, treat the switch with caution.
Can I ask my pharmacy to always give me the same generic brand?
Yes. You can request a “non-substitutable” prescription or ask your doctor to write “Dispense as written” or “Do not substitute” on your prescription. Insurance may still try to switch you, but you have the right to push back. Many patients on NTI drugs choose to pay a little more out of pocket to avoid the risk of switching.
I switched generics and feel worse. Should I change my dose myself?
Never adjust your dose without talking to your doctor. Feeling worse could mean your levels dropped too low - or rose too high. Both can be dangerous. For example, low levothyroxine can cause fatigue and weight gain; too much can cause heart palpitations. Call your doctor, get a blood test, and let them decide if a dose change is needed.
Are newer generics safer than older ones?
Not necessarily. But some manufacturers are making higher-quality versions of NTI drugs with tighter controls. For example, Teva’s TacroBell tacrolimus has been shown to have less variability than standard generics. If your doctor or pharmacist recommends a specific generic brand because it’s more consistent, it’s worth sticking with it - even if it costs a bit more.
Will insurance cover the brand-name version if I need it?
Sometimes. If you can prove that switching generics caused health problems - like hospitalization, abnormal lab results, or loss of function - your doctor can file a prior authorization request. Many insurers will approve the brand-name drug if there’s documented clinical need. Keep records of your labs and symptoms to support your case.
Write a comment
Your email address will not be published.



11 Comments
So let me get this straight - we’re trusting life-or-death meds to companies that can’t even make the same pill twice without throwing people into the ER? 🤦♀️ I get cost savings, but this is like swapping your parachute brand because it’s cheaper. Thanks, capitalism.
Also, why is my pharmacist the only one who knows which generic I’m getting? Shouldn’t the label say ‘WARNING: THIS PILL COULD KILL YOU IF YOU’RE ON WARFARIN’? Just a thought.
Guys, I’ve been a pharmacist in Mumbai for 18 years and let me tell you - this isn’t even the half of it. In India, generics are made in small labs with zero QA, and sometimes the active ingredient is just… missing. I had a patient on phenytoin who switched to a $2 generic and had a seizure within 48 hours. The pill had 0.3% of the drug. Zero. Three. Percent. FDA’s 80-125% range is a joke when you’re dealing with third-world manufacturing. And don’t even get me started on how Indian generics get exported globally under different labels. The FDA doesn’t inspect 90% of these factories. I’ve seen the reports. It’s terrifying. You think this is bad in the US? You ain’t seen nothing yet. 😅
Doctors are just being lazy. If they actually monitored patients properly, we wouldn’t need all this drama. Just check INR once a year like you’re supposed to. Problem solved. But nope - now we’re blaming generics because no one wants to do their job.
Also, ‘non-substitutable’? That’s just a fancy way of saying ‘I’m too cheap to pay for brand.’
There’s a critical distinction here between bioequivalence and therapeutic equivalence - and the FDA’s current 80–125% range is fundamentally inadequate for NTI drugs. The pharmacokinetic variability isn’t just statistical noise; it’s clinically significant. For tacrolimus, even a 10% shift in Cmax can precipitate acute rejection. Studies from the American Journal of Transplantation (2021) show a 3.7x higher risk of graft loss when switching between uncontrolled generics. The proposed 90–111% range is a step forward, but we need batch-to-batch consistency tracking - like what’s done in the EU with the EMA’s ‘reference product’ designation. Until then, clinicians are forced into reactive dosing, not proactive safety.
And yes - this is why we document every switch in the EHR and auto-flag TSH/INR follow-ups. It’s not paranoia. It’s pharmacovigilance.
Oh wow. So the system is broken, but the solution is to make patients do more work? Thanks for the reminder that my health is now my full-time job.
Also, ‘ask your pharmacist which generic you’re getting’ - as if they’re not pressured to switch me every time the rebate changes. Like, sure, I’ll ask. Then I’ll get a 30-second ‘uh, we don’t track that’ and a new bottle with a different logo. Thanks for nothing, system.
I really appreciate this breakdown. I’m on levothyroxine and switched generics last year - felt like crap for weeks but thought it was just stress. Turns out my TSH was through the roof. Got it checked, dose adjusted, and now I’m fine.
Just wish I’d known sooner. This should be standard info on every prescription bottle. Like a little sticker: ‘NTI DRUG - CHECK LABS IN 6 WEEKS’.
For anyone on NTI meds: keep a symptom journal. I use a Notes app - date, pill brand, energy level (1–10), heart rate, sleep quality. When I switched from Mylan to Teva levothyroxine, my energy dropped from 8 to 3. I showed my endo the log. She immediately ordered a TSH. It was 8.2. We adjusted. Done.
You don’t need to be a doctor to notice patterns. Your body tells you. Just listen.
Also - ask for the brand if you can afford it. It’s not selfish. It’s self-preservation.
Okay, but what if this is all a big pharma scam? 🤔
Think about it - brand-name companies make a killing on their patents, then when generics come in, they quietly fund the ‘danger’ narrative to scare people into paying more. The FDA’s new 90–111% rule? Coincidence that Teva’s ‘TacroBell’ just happened to be the only one that fits it perfectly? And who owns Teva? Big Pharma. Who profits from ‘non-substitutable’ prescriptions? Big Pharma.
They need you scared. They need you paying $400 for a pill that costs $2 to make. I’m not saying this is true… but I’m not saying it’s not either. 🕵️♂️
It is, regrettably, a matter of considerable concern that the regulatory framework governing bioequivalence for narrow therapeutic index pharmaceuticals remains insufficiently stringent. The current 80–125% acceptance range, while statistically permissible, is demonstrably suboptimal in clinical practice, particularly when considering the pharmacokinetic variability inherent in certain generic formulations. The absence of mandatory therapeutic monitoring protocols across primary care settings further exacerbates the risk profile for vulnerable patient cohorts. It is, therefore, imperative that healthcare providers, pharmacists, and policymakers collaborate to implement a more rigorous, evidence-based standard - one that prioritises patient safety over fiscal expediency.
so like... if i switch my thyroid pill and feel like a zombie... its not me being lazy its the pill?? mind blown 🤯
Bro I switched from one generic to another for my seizure med last year and had a minor seizure at work. Didn’t tell anyone for weeks cause I thought I was just stressed. Then I found this thread. Got my levels checked - dropped 25%. Now I pay extra to stick with the same brand. Worth every penny.
Also, if you’re on one of these meds - DM me. I made a spreadsheet of which generics are actually decent. No joke.